The Iodine atom has 7 valence electrons. F also has 7 valence electrons. But since there are 5 atoms of F, we multiply 7×5= 35 valence electrons. Adding both we get 35+7= 42. Name: Iodine Symbol: I Atomic Number: 53 Atomic Mass: 126.90447 amu Melting Point: 113.5 °C (386.65 K, 236.3 °F) Boiling Point: 184.0 °C (457.15 K, 363.2 °F) Number of Protons/Electrons: 53 Number of Neutrons: 74 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 4.93 g/cm 3 Color: blackish Atomic Structure. Iodine should only have 7 valence electrons. For the non-transition elements (The 'A' groups) the number of valence electrons is equal to the group the element is in, Iodine is in group VIIA, so it.
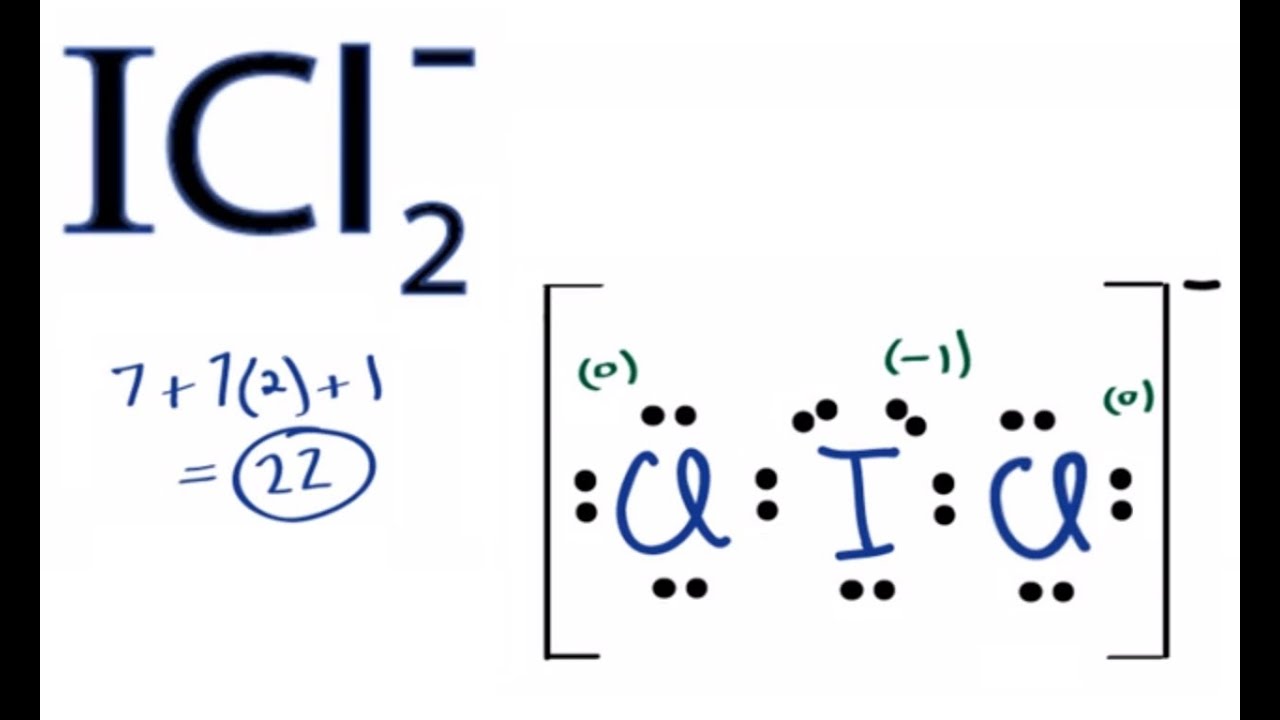
In group 17 how many valences electrons does Iodine have? How many valence electrons does Chlorine have?
1 Answer
Explanation:
This may seem a bit confusing when one looks at the periodic table, since chlorine is the seventh element in its row, but iodine is the seventeenth.
However, in the case of iodine, ten of the electrons that were added as we moved across the fifth row of the table belong to the fourth shell (the same one that is the valence shell for bromine), and not to the fifth.

Iodine Has Valence Electrons
The reason for this is rather lengthy, but suffice to say that it gives rise to the transition metals - these ten elements that make up columns 3 through 12. The electrons are filling energy levels based on the lowest available energy (as opposed to furthest from the nucleus, which is not quite the same thing).
Because these ten electrons are not in the outermost shell, they are not valence electrons.
Specifically, with iodine, the fifth shell begins with two electrons, then ten go into the fourth shell as already mentioned, before the next five occupy the fifth shell again, for a total of seven valence electrons (just like chlorine).
Iodine Valence Electrons
Related questions
